NK cell therapy
In recent years, NK cell therapy has been rapidly advancing in clinical applications for cancer, autoimmune diseases, infectious diseases, and Alzheimer’s disease. In the past, the inability to effectively obtain NK cells with high purity and sufficient quantity hindered the progress of cell therapies. However, with advancements in cell sorting technology, breakthrough NK cell expansion techniques have emerged, making NK cells a viable treatment option. NK cell-based immunotherapy can be widely applied to the treatment of various cancers, with allogeneic NK cells showing the best response in hematologic cancers (such as lymphoma, leukemia, etc.). When combined with standard therapies like targeted monoclonal antibodies, the cancer-fighting advantages of NK cells can be further enhanced. In addition, CAR-NK, which combines CAR gene modification technology, is also rapidly developing. It holds the potential to overcome the challenges of applying cell therapy to solid tumors, making allogeneic cell therapy a clinically routine ‘living drug.
ApexNK® Proprietary Technology Platform
A science-driven NK and CAR-NK platform engineered with quality by design.
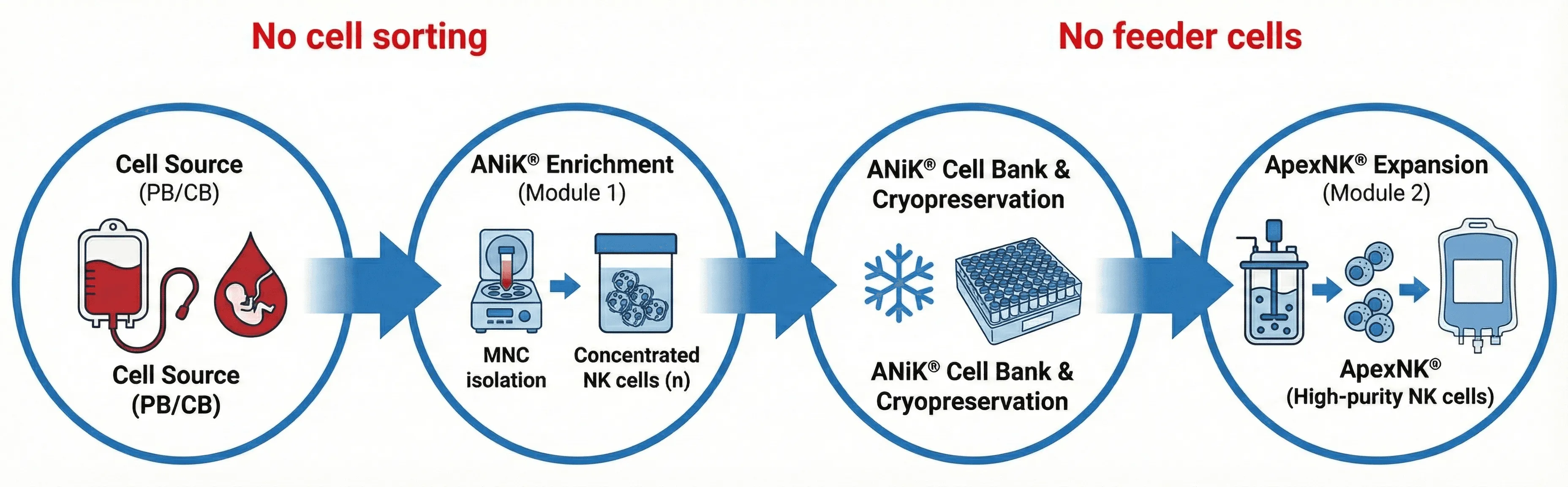
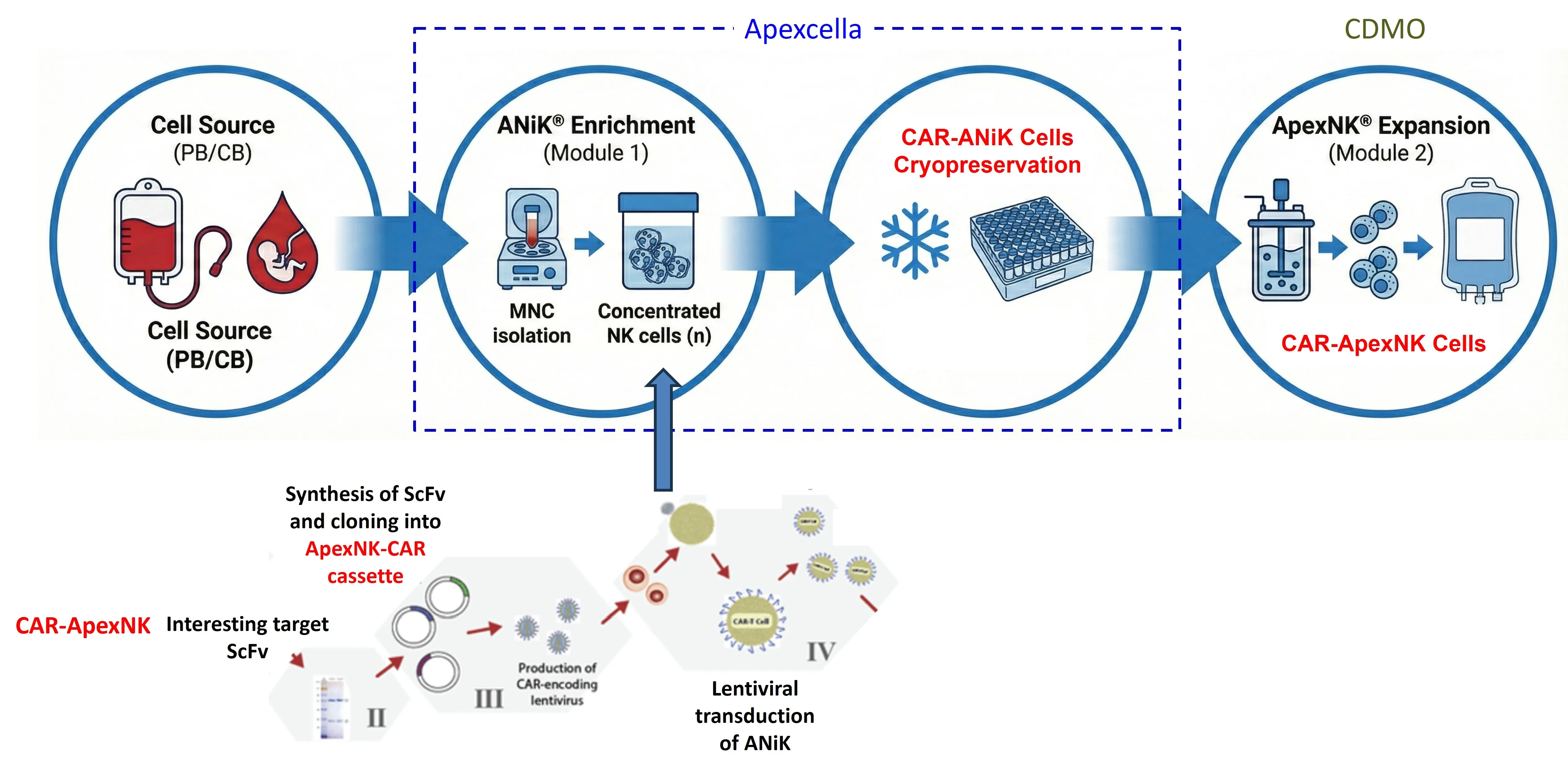
Apexcella’s modular ApexNK® cell expansion technology platform is the world’s first process design that enables industrial-scale, GMO-compliant production of clinical-grade ApexNK® cells. Without the requirement of cell sorting, the upstream process allows direct expansion of mononuclear immune cells from peripheral blood or cord blood into an NK cell-enriched intermediate stage called ANiK® cells, which are able to establish ANiK® cell bank for long-term storage. In the downstream process, the customized ApexNK® M2 kit enables thawed ANiK® cells to be directly expanded into high-purity ApexNK® cells, with both yield and cell activity meeting clinical requirements, in a closed system. In addition, by integrating a pseudotypic lentiviral transduction technology platform, a CAR-ANiK® cell bank can also be established, which can be paired with a closed-system process for large-scale production of CAR-ApexNK® cells.
Apexcella’s proprietary ANiK® cell bank and modular ApexNK® cell technology platform not only enable industrial-scale manufacturing but also provide strong global competitiveness and partnership opportunities. By leveraging long-term cryopreservation of ANiK® cells, storage space requirements are greatly minimized, while downstream processing can be seamlessly executed at CDMO facilities worldwide—fulfilling our vision of maintaining core manufacturing in Taiwan and driving a coordinated global commercial deployment. (US provisional patent 63/765,968)
Apexcella is focused on the allogeneic ApexNK® and CAR-ApexNK® cell technology platform and plans to leverage strategic alliances to integrate domestic and international industrial chains. Collaborating with academic institutions and biotech companies, Apexcella aims to develop innovative CAR-ApexNK® cell products targeting cancer, offering novel cell therapy solutions for solid tumors. The goal is to make this a standard therapy that is affordable and accessible to all patients in need. Through these efforts, Apexcella hopes to open the door to allogeneic NK cell therapy.
ANiK® cell bank
The ANiK® Cell Bank is currently the world’s only NK cell-enriched intermediate product that enables long-term cryopreservation, directly expanded from peripheral blood or cord blood mononuclear cells without the need for prior cell sorting. Compared with cryopreserved NK cell final products, it requires significantly less storage space, effectively reducing cryopreservation costs. Using the downstream ANiK® M2 kit, each ANiK® cell unit can generate at least 1 billion ApexNK® cells, with highly consistent quality, quantity, and activity across production batches.
Applications of the ANiK® Cell Bank:
For cancer patients undergoing chemotherapy or radiotherapy, immune cells in better condition can first be prepared as an autologous ANiK® cell bank before treatment. This prevents the inability to obtain sufficient autologous NK cells due to immune function decline caused by treatment side effects.
In the future, ANiK® cells can be thawed under physician guidance to prepare autologous NK cells, which can then be combined with targeted antibodies or other therapies, without the need for additional blood collection.
Each batch of the ANiK® cell bank demonstrates a high level of quality consistency, ensuring stability and uniformity across individual cell product doses in subsequent autologous NK cell therapies.
By integrating with the pseudotypic lentiviral transduction platform, ANiK® cells can further be prepared into a CAR-ANiK® cell bank. This approach requires only minimal viral vector input and can subsequently be combined with the ANiK® M2 kit to produce CAR-ANiK® cells in a closed system.
ApexNK® M2 kit
Contents of ApexNK® M2 kit:
Components
Catalog No.
Volume
Quantity
NK Medium
RMBNKR500M
500mL
1 BT
NK Medium Supplement
RMBNSR005M
5mL
1 PC
HR-1 Supplement
RACH1G050M
50mL
1 PC
EM1, 2, 3 Supplement
SSPEMX040U
40uL
6 PC
EM4 Supplement
SSPEMX090U
90uL
2 PC
Cell Culture Bag
CCL-0005-G1
500mL
2 PC
Competitive Advantages of the ApexNK® Proprietary Technology Platform
Advantages of the ApexNK® Proprietary Technology Platform:
Highest Process Feasibility – Applicable to both peripheral blood and cord blood samples; requires as little as 30 mL; no cell sorting needed; fully compliant with GMP guidance.
Maximum Product Safety – No feeder cells or animal-derived reagents used; final cell products are free from external contamination risks.
Highest Expansion Capability – Enables over one-million-fold expansion, achieving industry-scale production capacity.
Built-in Cryopreservation Design – With the ANiK® cell bank for stable cryopreservation, the process can be divided into upstream and downstream stages; reduces storage space needs, lowers costs, and enhances stability and consistency.
Highest Product Quality – ApexNK® cells achieve an average purity of over 95%, viability above 98%, and cytotoxicity exceeding 90% against K562 cells, demonstrating excellent NK cell killing activity.
Lowest Production Cost – With hundred thousand- to million-fold expansion capability and optimized process design, overall manufacturing costs can be significantly reduced.
CAR-ApexNK® Technology Platform
Apexcella has mastered the key CAR-ApexNK® cell manufacturing technology. By utilizing a specialized pseudotypic lentiviral vector in combination with the ApexNK® expansion process, an average transduction efficiency of 70% - reaching as high as 88.5% - can be achieved. These cells can be further prepared as CAR-ANiK® cell bank for long-term stable cryopreservation. In downstream processes, CAR-ApexNK® cells can be produced in closed-system bioreactors and exhibit excellent cell viability, expansion capacity, and tumor-killing activity. Currently, through strategic alliances with domestic academic institutions and biotech companies, Apexcella is pursuing the development of innovative CAR-ApexNK® cell therapies targeting cancer.